CHEM460A
Fall 2009
Welcome to Physical Biochemistry! Our first week topics are listed here, and I will post updates or revisions to the course topics and material here throughout the semester.
Week 1: Chapters 1 & 2 (week1.pdf)
No Lab
8/31: Thermodynamics review
-enthalpy, entropy, free energy, spontaneity
9/02: Equilibrium and standard state, deltaG vs. deltaG°’
Example problem 1 (Concentration_Activity.pdf)
Example problem 2: Calculate the standard enthalpy, entropy and free energy changes for combustion of glucose (C6H12O6) given the following information:
C6H12O6 (s): Hf(not)=-1273.3 kJ/mol, S(not) = 212.1 J/mol K
O2 (g): Hf(not) = 0, S(not) = 205
CO2 (g): Hf(not) = -393.5, S(not) = 205
H2O (l): Hf(not) = -285.4, S(not) = 69.9
What is the Keq? Is the forward or reverse reaction spontaneous? Is it enthalpically or entropically driven?
Properties of Water, Intermolecular forces
9/04: continued
Week 2: Chapter 2 (week2.pdf)
Lab: Experiment 1, Check-in, pipetting/buffers
**All Monday lab students must attend lab during another section this week.**
9/07: no class (Labor Day)
9/09: Quiz 1, Acids/Bases
Key posted, grades on Blackboard
9/11: Acids/Bases, practice problems
Week 3: Chapters 4 & 8 (week3.pdf)
Homework assignment 1 (VOLUNTARY) (HW1_2009.pdf). If you turn it in, it will be part of your HW/Quiz grade. It should be great practice applying thermodynamics and acid-base chemistry to amino acid structures.
Lab: Experiment 2, pKa of 4-nitrophenol
9/14: Amino Acids & Sugars
9/16: R/S, sugars
Notes: 1) We need to consider how the electronic environment of an ionizable group affects its pKa. For example, does a nonpolar solvent raise or lower the pKa of a carboxylic acid?
-
2)Most sugars have D stereochemistry, because this places the CH2OH in the equatorial position.
-
3) Along the same line, the beta-glucose places the anomeric OH in the equatorial position, which is more stable than in the alpha (axial) position. This is why the beta anomer is more populated.
-
4)Correction from today’s lecture: The proR prochiral proton is the proton you can see and rank, NOT the one you push to the back. More on this Friday!!!!!!!
9/18:
QUIZ 2 NOTICE!!!
For Monday, please make sure you are prepared for the following:
Amino acid sidechains and properties
Be able to draw an amino acid with D/L stereochemistry
Sugar topics
Be able to draw a sugar with D/L stereochemistry
Week 4: Chapters 3, 23 & 9 (week4.pdf)
Lab: Fumarase1
9/21: Quiz 2, Lipids & Nucleic Acids
NOTES for Exam 1 so far (I will also include some other topics from your notes on the exam).
Exam 1 topics: buffer question, amino acid structures and properties (amide, imidazole hydrogen bonding (lone pairs are conjugated, so they are unavailable for accepting H-bonds)), thermodynamic equilibria (acid-base, enthalpy, entropy, free energy, electrostatic environment, sign of energy change!!!!!!!!!!!!!!!!!, heat is evolved when a bond is made (deltaH < 0)), isoelectric point and net charge. If you are at standard state conditions and your ΔG°’ is -4kJ/mol, will the reaction proceed forward or backward? Given the concentrations of reactants and products at equilibrium, can you calculate the DG°? If you are given the ΔG° and current concentrations, can you tell if the forward or reverse rxn is spontaneous, and the ΔG? Energetics of electrostatic interactions (approximate magnitude of each type), and importantly, can you list all the contributing terms in the entropy and enthalpy change, along with sign (which interactions are made or broken during a process, and what is the sign of that term?).
Amino acid stereochemistry. Sugar stereochemistry (D/L, beta/alpha), glycosidic linkages, polysaccharides, reducing ends. Fat shape, function, thermodynamics (cis/trans unsaturated fatty acids relative properties).
9/23:
9/25:
Week 5, Chapter 6 (week5.pdf)
Lab: Fumarase2
9/28: wrap-up, review concepts
9/30: Exam 1 (evening)
10/02: Peptides and Proteins
Week 6, Chapter 6 (week6.pdf)
Lab: Fumarase3/Writing workshop
10/05: Protein structure and stability
10/07: X-ray crystallography (X-ray.ppt)
10/09: NMR and protein stability (NMR.ppt)
Week 7, Chapter 6 & 5 (week7.pdf)
Lab: Modeling Protein Structure
10/12: Quiz #3, protein folding
10/14:
10/16: Protein folding (Folding.ppt)
Week 8, Chapter 5 (week8.pdf)
No Lab
10/19: no class, fall break
10/21: Protein purification
10/23: Protein sequencing techniques
Week 9, Chapter 7 (week9.pdf)
Lab: Purification of Lysozyme
10/26: Quiz 4, Protein sequencing
10/28: Protein-ligand interactions
10/30:
Friday, Oct. 30 list of topics for Chem460 studying:
Recognizing Phi/Psi angles, forces holding proteins together, entropy of polypeptide backbone trying to unfold the protein, Hydrogen bonding within secondary structure elements (distances, what binds to what), NOESY (protons within 5Å give a crosspeak), COSY (protons directly connected with three bonds give a crosspeak), NMR structure properties vs. X-ray crystallography structure properties, Circular dichroism is observed as a weighted sum of all secondary structure present, contributions to protein stability and your ability to draw free energy diagrams for given proteins and their mutants, denaturants, folding steps and rates of 2° vs. 3° vs. 4° structure formation, measurable properties of each type of structure, properties of a mis-folded protein, propensity to mis-fold, ability of a protein with some random coil to then adopt amyloid structure (even if the natively structured protein does not form amyloid), PDI and GroEL/ES, salt and protein stability, salting in, salting out, ion exchange chromatog vs. pH and pI, size exclusion chromatog, affinity chromatog, hemoglobin and myoglobin (fractional saturation (YO2), cooperative protein structural changes, sigmoidal vs. hyperbolic curves, hill coefficient, bohr effect.
Week 10, Chapter 11 (week10.pdf)
Lab: Purification of Lysozyme2
11/2: Cooperativity, BPG, High altitude
11/4: Exam #2 (evening)
11/6: Enzymes, reaction diagrams
Week 11, Chapter 11 (week11.pdf)
Lab: Lysozyme3, Mass Spectrometry of Proteins
11/09: Enzyme tricks
11/11: Catalytic mechanisms: Serine proteases
11/13:
Week 12, Chapter 12 (week13.pdf)
Lab: Kinetic Analysis of Lactase
11/16: Quiz #5, Lysozyme mechanism (Vocadlo paper)
11/18: Michaelis-Menten kinetics
11/20:
Week 13, Chapter 12
No Lab
11/23: Inhibitors
11/25: TG travel day
11/27: Thanksgiving Holiday
Week 14, Various readings (week14_15.pdf)
Lab: Kinetic Analysis of Lactase2
11/30: Quiz #6 (NOTE: Instead of Quiz #6, HW#2 is due 12/2!!!!!), Arachidonic acid pathway
12/02: Drug Development
12/04:
5:15 pm review session: List of study topics. This list is not all-inclusive. Note: I will include examples from your class notes, as well, so make sure you have reviewed in your notes what I actually mentioned in class.
Exam 3 study-guide topics:
MS, reaction diagrams (know how to determine ∆G‡, ∆∆G‡cat, ∆G° on a plot), How does tight substrate binding influence the appearance of the reaction energy diagram? Enzyme tricks used to reduce energy of ES‡. Relative affinity of a transition state analog and the substrate. Rate enhancement and ∆∆G‡cat. Rate enhancement and relative affinity for ‡ vs. S.
Serine protease mechanism, substrate specificity (pocket), reactive residues in active site (catalytic triad, and know how each of the amino acids enhances the reaction rate). Role of Oxyanion hole in catalysis.
Lysozyme mechanism, rate of two steps in reaction and how they were manipulated in the Vocadlo and Withers paper. Structure of lysozyme transition states. Distortion of D sugar ring: energetics and its role in catalysis.
Reaction kinetics: zero-order vs. first order vs. second order (specifically, concentration dependence of reaction rates)
Rate constants for individual lysozyme reaction steps.
Michaelis-Menten equation, assumptions.
Definition and units of KM and Vmax. Conceptual understanding of KM and Vmax and what might influence each.
Effect of the different types of inhibitor on KM and Vmax.
Enzyme efficiency and the diffusion-controlled limit.
Shape of Michaelis-Menten plot for different types of inhibition.
Lineweaver-Burk plot for different types of inhibition: can you determine KI and inhibitor type from the plot?
Bisubstrate mechanism type for lysozyme, serine proteases, RNAse A.
Week 15, Wrap-up and review
No Lab
12/07: Wrap-up
12/08: Exam #3 (evening)
Friday, 12/18 Final Exam 8:00am-10:00am
Notes
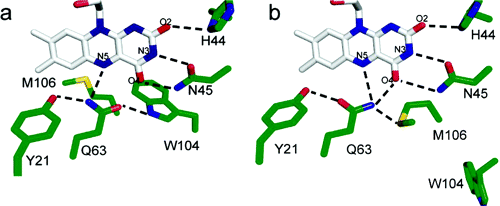
Dark state crystal structures of AppA a) WT and b) C20S mutant. The sidechain of Q63 rearranges upon absorption of a blue-light photon by the flavin cofactor, causing a structural change in the protein that alters its interaction with the transcriptional regulator protein PpsR. From Grinstead, et al., J. Am. Chem. Soc., 2006, 128 (47), pp 15066-15067.
Course Information