

Refractometry:
Analyzing Results
Finding Refractive Indexes
One of the most common uses of the refractive index is to compare the value you obtain with values listed in the literature. This comparison is used to help confirm the identity of the compound and/or assess its purity. The following sources list refractive indexes for a wide variety of substances:
The CRC Handbook of Chemistry and Physics
Chemical catalogs (e.g., the one from Aldrich Chemical Co.)
MSDS datasheets (many are available on the web)
There are also many computer-based chemical databases that contain refractive indexes. For example, both the CRC Handbook of Chemistry and Physics, and The Merck Index have computer-based versions. These can be particularly useful if your sample is an unknown and you want to search for compounds with similar indexes of refraction. One of the most comprehensive databases for organic compounds is MDL's Beilstein Crossfire database. (Last time I checked it contained 96 reported values for the index of refraction of isopropanol alone!) If you don't have access to one of the commercial chemical databases, I recommend The Organic Compounds Database at Colby College which can be used on the web for no charge.
Comparing Refractive Indexes
Since the refractive index of a substance depends on the wavelength it is important that the refractive index you are comparing to was obtained at the same wavelength as the one you determined. This is usually not an issue since the vast majority of refractive indexes are obtained using the sodium D line at 589.3 nm. (Even refractometers that use white light are normally constructed so that the refractive index obtained corresponds to that for light at 589.3 nm.)
The refractive index also depends on the temperature. Thus, it is best to obtain the refractive index of your sample at the same temperature as the value you plan to compare with; in most cases this will be 20 °C. However, if your refractometer is not equipped with a temperature regulating system, you may simply be stuck with room temperature, whatever that happens to be.
For most organic liquids the index of refraction decreases by approximately 0.00045 ± 0.0001 for every 1 °C increase in temperature. See Table 1 for a few examples. Note that the index of refraction for water is much less dependent on temperature than most organic liquids, decreasing by about 0.0001 for every 1 °C increase in temperature.
| Substance | |||
| Isopropanol | 1.3802 | 1.3772 | 1.3749 |
| Acetone | 1.3616 | 1.3588 | 1.3560 |
| Ethyl Acetate | 1.3747 | 1.3742 | 1.3700 |
| Water | 1.3334 | 1.3330 | 1.3325 |
If you determined your index of refraction at a different temperature than that reported in the literature you will need to correct your value for the temperature variation before comparing it to the literature value. For example, if you determined the index of refraction of an organic liquid at 24°C, and want to compare it to a literature value determined at 20 °C, you should subtract 4(0.00045) = 0.0018 from the index of refraction you obtained.
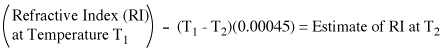 |
| Figure 1. Equation for estimating the index of refraction at a temperature different than that used for the measurement. This method relies on the observation that the temperature variation in the index of refraction is similar for many organic liquids. This correction is only approximate and should not be used for aqueous solutions. |
A typical laboratory refractometer can determine the refractive index of a sample to a precision of ± 0.0002. However, small amounts of impurities can cause significant changes in the refractive index of a substance. Thus, unless you have rigorously purified your compound, a good rule of thumb is that anything within ± 0.002 of the literature value is a satisfactory match.
Another possible source of error is miscalibration of the refractometer. This is readily checked by using a sample of known refractive index. Distilled water is a particularly convenient standard since it is nontoxic, readily available in pure form, and its refractive index varies only slightly with temperature (Table 1). If you find that the index of refraction of the standard is consistently off by more than 0.0005 from the expected value report this to your instructor or the person in charge of calibrating the refractometer.
Probably the most common source of error in analog refractometers is misreading of the scale. If the index of refraction you determined seems inconsistent with other data, try repeating the measurement.
Determining Concentrations of Solutions
Determining the concentration of a solute in a solution is probably the most popular use of refractometry. For example, refractometer-based methods have been developed for determining the percentage of sugar in fruits, juices, and syrups, the percentage of alcohol in beer or wine, the salinity of water, and the concentration of antifreeze in radiator fluid. Many industries use refractometer-based methods in quality control applications.
In most cases the refractive index is linearly (or nearly linearly) related to the percentage of dissolved solids in a solution (Figure 2). By comparing the value of the refractive index of a solution to that of a standard curve the concentration of solute can be determined with good accuracy. Many refractometers contain a "Brix" scale that is calibrated to give the percentage (w/w) of sucrose dissolved in water.
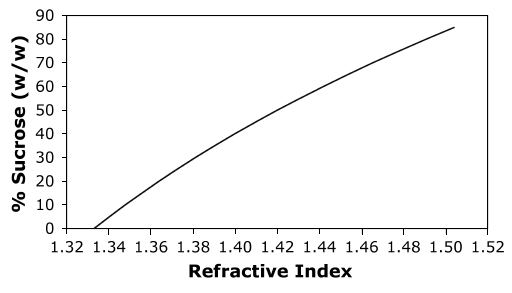 |
| Figure 2. A standard curve showing the relationship between the refractive index and the percentage (w/w) of sucrose in a solution of water at 20 °C. A much more detailed standard curve for the relationship between the refractive index and the percentage of sucrose is available as a PDF file.) |
Structural Information
The refractive index does not provide detailed information about a molecule's structure, and it is not usually used for this purpose since spectroscopic techniques are much more powerful at revealing details of molecular structure. One structural factor that influences the refractive index of a sample is its polarizability. Substances containing more polarizable ("soft") groups (e.g., iodine atoms or aromatic rings) will normally have higher refractive indexes than substances containing less polarizable ("hard") groups (e.g., oxygen atoms or alkyl groups). See Table 2 below.
Substance |
2-Iodoethanol |
2-Fluoroethanol |
Benzene |
Cyclohexane |
 |
1.5720 |
1.3670 |
1.5010 |
1.4260 |
