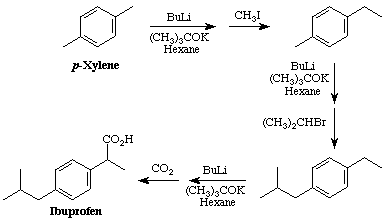
This strategy uses the "superbasic" mixture of butyllithium and potassium tert-butoxide to deprotonate benzylic positions. These anions can then be used as nucleophiles. Using this strategy, p-xylene was sequentially reacted with methyl iodide, isopropyl bromide, and carbon dioxide to yield ibuprofen in 52% overall yield.

The table below lists the chemicals that we will have available for this project. If you need something that is not on this list, consult with the mentor for your project. Also note the "Amount/group" column. This is the total amount of material available for each group to use on the project.
| Reagent | Source | Amount/group | Location | Comments |
|---|---|---|---|---|
| p-Xylene Anhydrous |
Aldrich Cat. # 29,633-3 |
20 g | TA room | |
| Iodomethane | Malinckrodt Cat. # 1077 |
10 g | Th 303? Special storage cabinet |
Highly Toxic! Cancer Suspect Agent! Handle with gloves in a hood. |
| 2-Bromopropane | Aldrich Cat. # B7, 811-4 |
10 g | TA room | Flammable Solid, Moisture Sensitive |
| Butyllithium 1.6 M solution in Hexanes |
Aldrich Cat. # 18,617-1 |
20 mL | TA Room | Flammable liquid. Moisture sensitive. Dispense under nitrogen. |
| Potassium tertbutoxide | Aldrich Cat. # 15,667-1 |
10 g | TA room | Flammable solid. Moisture Sensitive. |
A mixture of p-xylene (20 mmol), butyllithium (22 mmol), and potassium tertbutoxide (22 mmol) in hexane (15 mL) was vigorously stirred 2 h at 25 ° C. Methyl iodide (22 mmol) was introduced dropwise, in the course of 5 min, at -75 ° C. .... Then let warm to room temperature. They then went on to add more superbase and continue, but we should probably just isolate this material and see if the reaction works for us.