PENGUIN (Neshyba, Rowe, & Rivers, 2023)¶
Warming of an ice-free Arctic¶
Introduction¶
Over the last few decades, the temperature of the Arctic region has been rising fast -- at a rate of about 1 degree per decade (see https://climate.copernicus.eu/esotc/2020/arctic-temperatures). That's as much as four times faster than the rest of the Earth has been warming (see https://www.nature.com/articles/s43247-022-00498-3). This extra warming, called Arctic amplification, is thought to be due primarily to the reduction in the coverage of ice and snow in the Arctic region, over land and sea.
The temperature difference you'll calculate here is all about an additional kind of Arctic amplification due to the complete disappearence of summertime ice and snow over the Arctic Ocean. Arctic amplification due to this mechanism hasn't fully kicked in yet because Arctic sea ice is still present in the summer, but we know it is happening -- you can see the trend in Figs. 1 and 2 below.
 Figure 1. The 2015 Arctic sea ice summertime minimum, compared to the 1981-2010 average (from https://www.nbcnews.com/science/environment/rapid-melt-arctic-sea-ice-shrinks-fourth-lowest-level-record-n427866?cid=par-sy-_all).
Figure 1. The 2015 Arctic sea ice summertime minimum, compared to the 1981-2010 average (from https://www.nbcnews.com/science/environment/rapid-melt-arctic-sea-ice-shrinks-fourth-lowest-level-record-n427866?cid=par-sy-_all).
 Figure 2. Seasonal and interannual variation in Arctic sea ice areal extent (from https://zacklabe.com/arctic-sea-ice-extentconcentration/).
Figure 2. Seasonal and interannual variation in Arctic sea ice areal extent (from https://zacklabe.com/arctic-sea-ice-extentconcentration/).
Positive feedbacks and climate tipping points¶
Thinking about the relationship between the disappearance of sea ice and $CO_2$-induced global warming, you might make the following objection: isn't the disappearance of sea ice the result of an increase in temperature due to human emissions of $CO_2$? How can it be both cause and effect? The answer is, $CO_2$-induced warming and sea ice loss feed off one another, known in climate science as a positive feedback between the two processes.
Positive feedbacks are especially worrisome because embedded in them is the idea that at a certain point, these feedback mechanisms might lead to what is called a tipping point. Dynamical systems that experience positive feedback are susceptible to taking on a life of their own. One big worry regarding the Arctic is that it has already passed, or is at least very close to, a tipping point in which summertime Arctic sea ice will go away permanently, and therefore the warming will continue even if humans back off on the $CO_2$ that got the warming started.
There are lots of other ways the climate system could accidentally trigger tipping points when the global temperature rises above $1.5-2$ degrees Celsius -- for further reading, you can check out https://rmets.onlinelibrary.wiley.com/doi/full/10.1002/wea.4058. Avoiding these climate tipping points is a big reason the signers of the Paris Climate Agreement (https://unfccc.int/process-and-meetings/the-paris-agreement) stipulated that we should really, really keep warming under $1.5-2$ degrees Celsius.
The idea of this CGI¶
This CGI is an exploration of how much the Arctic's temperature would go up as a result of the additional loss of ice and snow in the Arctic Ocean. Figure 3 shows the factors we'll be considering here. The questions we'll be focusing on are: How much additional warming might there be? and How does that compare with the Arctic amplification we've seen so far?
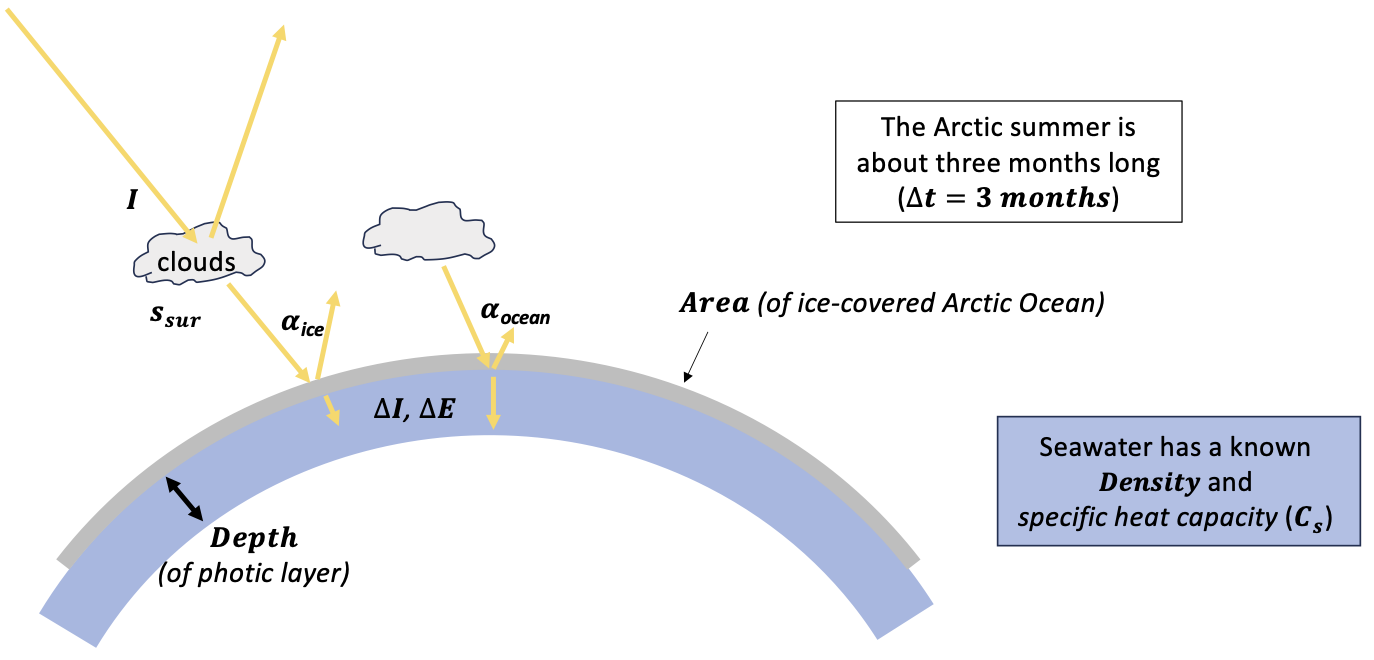 Figure 3. Factors influencing the heating effect of loss of summertime Arctic Ocean ice.
Figure 3. Factors influencing the heating effect of loss of summertime Arctic Ocean ice.
Part 1. Heat capacity of the photic layer of the Arctic Ocean¶
Where to start? You probably have an intuition about this: when you try to heat something up, the more massive it is, the harder it is to heat it up. In our case, we'll need the total mass of the photic layer of the ocean (top 200 meters) of the Arctic Ocean, since that's the layer that will be absorbing most of that additional sunlight once the cover of sea ice is gone.
$$ mass = Area \times Depth \times Density \ \ \ \ (1) $$Those quantities have been measured -- some by satellite, others in laboratories. Check out these sources for values:
- https://en.wikipedia.org/wiki/Arctic_Ocean
- https://www.noaa.gov/jetstream/ocean/layers-of-ocean
- https://en.wikipedia.org/wiki/Photic_zone
- https://en.wikipedia.org/wiki/Seawater
To get the heat capacity, $C$, of the photic layer, we can use
$$ C = C_s \times mass \ \ \ \ (2) $$where $C_s$ is called the specific heat capacity of water. That's also a known quantity (see https://en.wikipedia.org/wiki/Table_of_specific_heat_capacities).
Part 2. The insolation impact¶
We'll also need to know the difference in solar energy getting into the Arctic Ocean when the ice is gone. We can start with how much light is hitting the surface in the first place. That's called the insolation, and symbolized as $I$. Insolation varies with the time of year, and with the latitude, but that's all been worked out -- check out the 8th figure down at http://arctic-news.blogspot.com/2013/06/open-water-in-areas-around-north-pole.html, as an example.
Now, melting ice doesn't change $I$ -- that's controlled by the sun. But melting does change the fraction of $I$ that actually warms the surface. That's because sea ice reflects away a lot more sunlight than the open ocean does. This reflectivity has a special name in climate science: it's called the albedo. Albedos of all kinds of surface types have been measured -- see https://en.wikipedia.org/wiki/Albedo.
One more note about this -- there are clouds above the ocean in the Arctic that also reflect sunlight. What that means is, the sensitivity, $s_{sur}$, of the overall albedo of the Arctic to changes in the surface albedo, is small -- only about 11% (see https://www.mdpi.com/2073-4433/10/1/12).
So in the end, we have a total solar insolation difference given by
$$ \Delta I = (\alpha_{ice} - \alpha_{ocean}) \times Area \times s_{sur} \times I \ \ \ \ (3) $$where $Area$ is the same as what we've already considered, namely, the areal extent of the Arctic Ocean.
Part 3. The energy impact¶
Now we're ready to find the energy difference resulting from the extra sunlight being absorbed after the cover of snow and ice is gone. It's given by
$$ \Delta E = \Delta I \times \Delta t \ \ \ \ (4) $$where $\Delta t$ is the length of time the Arctic Ocean will be ice-free. If we say the entire summer is ice-free, we'd specify $\Delta t \approx 3 $ months.
Part 4. The temperature impact¶
A pretty straightforward thermodynamic analysis says that the temperature change we can expect when that additional energy is absorbed by the photic layer of the Arctic Ocean will be given by
$$ \Delta T = {\Delta E \over C} \ \ \ \ (5) $$Background reading¶
Useful background reading for this activity is https://e360.yale.edu/features/as_arctic_ocean_ice_disappears_global_climate_impacts_intensify_wadhams.
Learning goals¶
- I know the months of the year when Arctic sea ice extent is at a maximum and a minimum;
- I can explain how the following terms relate to climate: positive feedback, albedo, specific heat capacity, insolation, photic layer, Arctic Amplification, and tipping point;
- Given the mass and specific heat capacity of a substance, I can find its total heat capacity;
- Given the area and insolation of geographical region (like the Arctic), I can find the region's total insolation; and
- I can predict how much the Arctic is likely to warm when summertime ice disappears.