Poisons





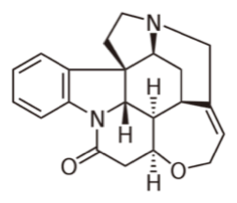
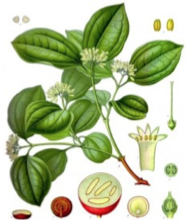
Strychnine is a toxic alkaloid found in the plant Strychnos nux vomica, a deciduous tree found in southeast Asia. It is a white, odorless, crystalline powder that his highly poisonous with a median lethal dose in humans of only 1-2 mg/kg when ingested orally. It is a convulsant that causes increased reflex excitably, causing all muscles to simultaneously contract, leading to death by asphyxia. Due to its toxic effects, strychnine is commonly used as a pesticide used to kill moles, birds, rats, and other pests. However, due to increasing numbers of accidental poisonings, most pesticides in the U.S. have replaced strychnine with zinc-phosphides.
The toxic effects of have been documented since the early 1800s in ancient China and India. French Chemists Joseph Bienaime and Pierre-Joseph Pelletier discovered strychnine in 1818 in the Saint-Ignatius’ bean (the seed of the tree Strychnos ignatia, which is closely related to Strychnos nux vomica). However, records show that strychnine may have been used to kill birds, cats, and dogs in Europe as early as 1640. Either way, it wasn’t until 1946 that strychnine’s structure was determined, and Robert Woodward synthesized it 8 years later. This synthesis project remains one of the most famous syntheses in the history of organic chemistry. Robert Woodward was awarded the Nobel Prize for his work.
Due to the complex structure of strychnine, its biosynthesis is quite complicated, and is only fairly well understood. It is derived from the enzymatic conversion (by strictosidine synthase) of tryptamine (chemically related to tryptophan) and secologanin (a monoterpene in the mevalonate pathway) to strictosidine, which is converted to strychnine though a series of six reactions with unknown enzymes.
Strychnine’s activity as a neurotoxin has been the topic of great interest over the course of history. The Nazis used strychnine to poison Jews during WWII, and the SS officers would watch the subsequent convulsive deaths for their personal entertainment. Strychnine affects the motor nerves in the spinal cord by blocking the receptors for glycine (an inhibitory neurotransmitter). These antagonistic properties of strychnine cause constant muscle contractions because glycine is unable to bind its receptor. This causes the inhibitory effect of glycine to be reduced, resulting in nerve impulses being triggered with decreased levels of neurotransmitters.
Strychnine has an active half-life of 10 hours. It is metabolized in the liver by a microsomal enzyme system. The short half-life of this compound suggests that the hepatic metabolism of strychnine can greatly aid in reducing the effects of ingestion of the poison. Nonetheless, in less than 20 minutes after oral exposure to strychnine, humans will start to experience muscle spasms. These spasms originate in the neck, but quickly spread to the rest of the body. These convulsions will intensify, causing the spine to continuously arch. Acidosis follows, soon before seizure and unconsciousness. Death occurs from asphyxiation within 3 hours. There is no antidote for the poison, but if caught early enough after ingestion, close medical attention can aid in recovery.
Strychnine is a toxic alkaloid found in the plant Strychnos nux vomica, a deciduous tree found in southeast Asia. It is a white, odorless, crystalline powder that his highly poisonous with a median lethal dose in humans of only 1-2 mg/kg when ingested orally. It is a convulsant that causes increased reflex excitably, causing all muscles to simultaneously contract, leading to death by asphyxia. Due to its toxic effects, strychnine is commonly used as a pesticide used to kill moles, birds, rats, and other pests. However, due to increasing numbers of accidental poisonings, most pesticides in the U.S. have replaced strychnine with zinc-phosphides.
The toxic effects of have been documented since the early 1800s in ancient China and India. French Chemists Joseph Bienaime and Pierre-Joseph Pelletier discovered strychnine in 1818 in the Saint-Ignatius’ bean (the seed of the tree Strychnos ignatia, which is closely related to Strychnos nux vomica). However, records show that strychnine may have been used to kill birds, cats, and dogs in Europe as early as 1640. Either way, it wasn’t until 1946 that strychnine’s structure was determined, and Robert Woodward synthesized it 8 years later. This synthesis project remains one of the most famous syntheses in the history of organic chemistry. Robert Woodward was awarded the Nobel Prize for his work.
Due to the complex structure of strychnine, its biosynthesis is quite complicated, and is only fairly well understood. It is derived from the enzymatic conversion (by strictosidine synthase) of tryptamine (chemically related to tryptophan) and secologanin (a monoterpene in the mevalonate pathway) to strictosidine, which is converted to strychnine though a series of six reactions with unknown enzymes.
Strychnine’s activity as a neurotoxin has been the topic of great interest over the course of history. The Nazis used strychnine to poison Jews during WWII, and the SS officers would watch the subsequent convulsive deaths for their personal entertainment. Strychnine affects the motor nerves in the spinal cord by blocking the receptors for glycine (an inhibitory neurotransmitter). These antagonistic properties of strychnine cause constant muscle contractions because glycine is unable to bind its receptor. This causes the inhibitory effect of glycine to be reduced, resulting in nerve impulses being triggered with decreased levels of neurotransmitters.
Strychnine has an active half-life of 10 hours. It is metabolized in the liver by a microsomal enzyme system. The short half-life of this compound suggests that the hepatic metabolism of strychnine can greatly aid in reducing the effects of ingestion of the poison. Nonetheless, in less than 20 minutes after oral exposure to strychnine, humans will start to experience muscle spasms. These spasms originate in the neck, but quickly spread to the rest of the body. These convulsions will intensify, causing the spine to continuously arch. Acidosis follows, soon before seizure and unconsciousness. Death occurs from asphyxiation within 3 hours. There is no antidote for the poison, but if caught early enough after ingestion, close medical attention can aid in recovery.
Strychnine is a toxic alkaloid found in the plant Strychnos nux vomica, a deciduous tree found in southeast Asia. It is a white, odorless, crystalline powder that his highly poisonous with a median lethal dose in humans of only 1-2 mg/kg when ingested orally. It is a convulsant that causes increased reflex excitably, causing all muscles to simultaneously contract, leading to death by asphyxia. Due to its toxic effects, strychnine is commonly used as a pesticide used to kill moles, birds, rats, and other pests. However, due to increasing numbers of accidental poisonings, most pesticides in the U.S. have replaced strychnine with zinc-phosphides.
The toxic effects of have been documented since the early 1800s in ancient China and India. French Chemists Joseph Bienaime and Pierre-Joseph Pelletier discovered strychnine in 1818 in the Saint-Ignatius’ bean (the seed of the tree Strychnos ignatia, which is closely related to Strychnos nux vomica). However, records show that strychnine may have been used to kill birds, cats, and dogs in Europe as early as 1640. Either way, it wasn’t until 1946 that strychnine’s structure was determined, and Robert Woodward synthesized it 8 years later. This synthesis project remains one of the most famous syntheses in the history of organic chemistry. Robert Woodward was awarded the Nobel Prize for his work.
Due to the complex structure of strychnine, its biosynthesis is quite complicated, and is only fairly well understood. It is derived from the enzymatic conversion (by strictosidine synthase) of tryptamine (chemically related to tryptophan) and secologanin (a monoterpene in the mevalonate pathway) to strictosidine, which is converted to strychnine though a series of six reactions with unknown enzymes.
Strychnine’s activity as a neurotoxin has been the topic of great interest over the course of history. The Nazis used strychnine to poison Jews during WWII, and the SS officers would watch the subsequent convulsive deaths for their personal entertainment. Strychnine affects the motor nerves in the spinal cord by blocking the receptors for glycine (an inhibitory neurotransmitter). These antagonistic properties of strychnine cause constant muscle contractions because glycine is unable to bind its receptor. This causes the inhibitory effect of glycine to be reduced, resulting in nerve impulses being triggered with decreased levels of neurotransmitters.
Strychnine has an active half-life of 10 hours. It is metabolized in the liver by a microsomal enzyme system. The short half-life of this compound suggests that the hepatic metabolism of strychnine can greatly aid in reducing the effects of ingestion of the poison. Nonetheless, in less than 20 minutes after oral exposure to strychnine, humans will start to experience muscle spasms. These spasms originate in the neck, but quickly spread to the rest of the body. These convulsions will intensify, causing the spine to continuously arch. Acidosis follows, soon before seizure and unconsciousness. Death occurs from asphyxiation within 3 hours. There is no antidote for the poison, but if caught early enough after ingestion, close medical attention can aid in recovery.
http://www.ncbi.nlm.nih.gov/pubmed/12352624
http://www.bt.cdc.gov/agent/strychnine/basics/facts.asp
http://www.inchem.org/documents/pims/chemical/pim507.htm#SubSectionTitle:7.2.1 Human data
http://en.wikipedia.org/wiki/Glycine_receptor
http://en.wikipedia.org/wiki/Strychnine
"Tobacco, coffee, alcohol, hashish, prussic acid, strychnine, are weak dilutions; the surest poison is time."
- Ralph Waldo Emerson
Wednesday, April 25, 2012


Strychnine - Justin Erickson