Poisons





Rotenone is a compound found in the roots and stems of certain plants, particularly those in the genus Lonchocarpus or Derris, including the jicama vine. The roots contain 3-10% of rotenone along with smaller amounts of other rotenoids. It was first isolated by Emmanuel Geoffrey from a plant in the genus Lonchocarpus, after he studied in French Guiana where the plant was used as a fish poison by the indigenous people. Though the chemical killed the fish, it did not affect those who consumed the poisoned fish because it absorbs poorly in the human gastrointestinal tract. The lipophilicity of the compound allows it to penetrate the gills of fish, which provides easy access to the bloodstream causing death. Poisoned fish usually move to shallower water or come to the surface as their ventilation slows and they die from respiratory arrest within 24-36 hours. Contaminating a body of water with this compound allowed the easy collection of dead, edible fish downstream.
Rotenone can be used as a white powder, or in its colorless and transparent crystalline form. It is an odorless chemical that has been used both as a piscicide and an insecticide. The compound is unstable in light and heat, so it degrades quickly during the summer months. As an insecticide against aphids, it is fifteen times as toxic upon contact as nicotine. Up until 2005, rotenone was approved by the United States Department of Agriculture for use on “organic” produce. However, it was removed from the list of government-approved substances due to concerns about its toxicity; it was classified as moderately hazardous by the World Health Organization. It has also been used to remove unwanted fish, such as invasive species, from particular aquatic environments. However, human deaths due to rotenone poisoning are rare because its ingestion causes vomiting, ridding the body of the compound.
Rotenone acts as a toxin by interfering with the electron transport chain within the mitochondria. Specifically, it inhibits the transfer of electrons from Complex I (NADH ubiquinone reductase) to ubiquinone. This prevents the conversion of NADH into the usable cellular form of energy, ATP, halting cellular respiration.
Additionally, rotenone has been found to cause symptoms similar to those of Parkinson’s disease when injected into rats. These symptoms include bradykinesia (slowness in voluntary movement), tremors in hands and feet, poor balance, and an unsteady walk. The mechanism by which rotenone induces these effects is thought to be its stimulation of the apoptotic pathway in neuronal cells.
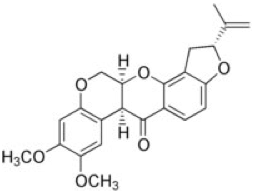
Rotenoids, the larger class of compounds of which rotenone is one example, are synthesized via a ring cyclization of a methoxyisoflavone derived from the shikimate pathway. Specifically, rotenone includes a C5 isoprene unit incorporated through a dimethylallylation step. Additionally, the isopropenylfurano system contained within rotenone is formed via a cyclization to a 5-membered ring from rotenoic acid. The structure of rotenone is shown below.
“Because there are niche uses and no direct replacements available for all piscicidal uses of rotenone, EPA concludes that [the continued use of rotenone] would provide benefit to society in controlling invasive or unwanted fish species.” –US Environmental Protection Agency, 2007
Wednesday, December 9, 2009


Rotenone






