Medicinal Plants





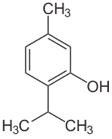
What do mummies and cigarettes have in common? They both contain the potent antimicrobial agent, Thymol, a terpene natural product found in thyme (Thymus vulgaris). Thymol is an essential oil whose first recorded uses were among the ancient Egyptians as a mummy preservative (along with carvacrol, another natural product derived from thyme) and the Blackfoot Native Americans who used it for skin infections and minor wounds. Thymol has also historically been used in the preservation of documents due to its ability to kill mold growth; however, thymol and its isomers have been found to turn yellow upon oxidation which is currently making thymol the bane of museum curators and archivists. Another seemingly random use of thymol is as a natural insect repellant in Afghanistan which may just be due to the strong odor of thyme oil.
Structurally, thymol is a monoterpene phenol and largely hydrophobic. The biosynthesis of the thymol follows the standard terpene route via geranyl phosphate and aromatic cyclization. The antibacterial and antioxidative properties of thymol and well as its relatively simple hydrogenation to menthol make it a popular compound for large scale synthesis. As thymol has a relatively simple structure the synthesis of it was discovered early. The first synthesis in 1882 by Widman was through a rather convoluted method that started with cuminal and ended with the diazotization of 3-aminocymene. Currently, the most popular synthetic route is the isopropylization of m-cresol which simply combines m-cresol and propylene (or isopropanol) and reacts them at high temperatures in the presence of various catalysts.
The most distinctive quality of thymol is its antimicrobial activity. Although little is known on the exact mechanism, it is believed that the hydrophobic properties of thymol may be an important property of the antimicrobial activity of thymol and its enantiomer, carvacrol. Both thymol and carvacrol act together synergistically to increase the antimicrobial activity, which gives evidence to the theory that plant based medicines have compounds that act in tandem to increase effectivenesss. These antimicrobial activities come from the fact that the hydrophobic property of terpenes allows them to enter the interior of cell membranes. The hydroxyl group of thymol creates an amphipathic molecule that after entering the membrane disrupts the fluidity. At high concentrations of thymol, these disruptions are enough to create holes in the membrane and completely disrupt the cell functions. The same effects cause some antifungal properties with the membrane disruption causing an inability of the fungus to bind to adhere to other cells. The antimicrobial properties of thymol have made it one of the lead ingredients in the mouthwash Listerine due to its ability to bacteria in the mouth that lead to bad breath. Carvacrol, a monoterpene structurally similar to thymol is found to have a synergistic effect with thymol in increasing antimicrobial activity which gives evidence to the theory that plant based medicine have synergistically working compounds.
Medicinally, thymol has been used for treating fungal infections of the feet which is once again due to the antifungal properties of thymol. Internally, thymol has been used for bronchitis, although the effects are unproven. Clinical studies have been performed to see if thymol has potential antitumor properties or antioxidant properties. Antioxidant properties have been found in rats treated with both thymol and thyme-oil where it was found that thymol and thyme-oil fed rats had higher antioxidant enzyme activities as they aged than untreated ants using either supplement. The antitumor properties of thymol are surprisingly abundant. In a study where leukemia cells where incubated with thymol for 24 hours it was found that thymol increases production of reactive oxygen species, mitochondrial hydrogen peroxide production, and depolarization of membrane potential only in cancerous cells. The cytotoxic effects were not seen in noncancerous cells. However, the amount of thymol necessary for these effects and its viability as a drug option is yet to be known.
The myriad of uses thymol has to offer and the relative simplicity of the molecule have made it a popular research topic. As a component in commercial products, thymol is ubiquitous and its medicinal applications are constantly being discovered. Thymol appears to have a vast future in chemical and medicinal research.
References
Ali, Asraf; Gaikar, Vilas. Microwave-assisted process intensification of synthesis of thymol using carbonized sulfonic acid resin catalyst. Ind. Eng. Chem Res., 2011, 50 (11), pp 6543-6555.
Braga, Pier Carlo; Ricci, Davide. Thymol-induced alterations in Candida albicans imaged by atomic force microscopy. Methods in Molecular Biology 2011;736:401-410.
Dutta Deb, Dipanwita; G. Parimala; S. SaravanaDevi. Effect of thymol on peripheral blood monomuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chemico-Biological Interactions. 2011:193(1), 97-106.
http://www.mendeley.com/research/yellowing-thymol-display-prints/
http://en.wikipedia.org/wiki/Thymol
Youdim KA, Deans SG. Effect of thyme oil and thymol dietary supplementation on the antioxidant status and fatty acid composition of the ageing rat brain. Br J Nutr 2000;83(1):87-93.
Friday, April 27, 2012


Thymol - Krista Thompson






