Medicinal Plants





Have you ever wondered what’s in your grandfather’s plethora of daily vitamins? Well, along with Viagra, there are probably a couple of white pills containing statins. Atorvastatin, a type of statin, which is manufactured by Pfizer under the name Lipitor, is a drug used to lower total and low-density lipoprotein (LDL) cholesterol levels and thus prevent cardiovascular disease. Statins lower cholesterol levels through inhibition of the enzyme HMG-CoA reductase, an enzyme which helps to produce cholesterol in the liver. Statins, which are part of a group of compounds called ketides, have terpene-like side chains and are able to inhibit the terpene synthesis of cholesterol. Statins have made a name for themselves in the U.S. pharmaceutical industry; according to IBIS World, Lipitor was the top-selling branded pharmaceutical in the world, with 2011 sales of US$ 5.3 billion. You might ask, what does this billion dollar drug have to do with natural products? The answer is in Penicillium citrinum, a fungus from which mevastatin, the first isolated statin, was found.
Mevastatin was discovered and identified as a HMG-CoA reductase inhibitor by Akira Endo in the 1970s. Although clinical trials on compactin (mevastatin) were performed shortly after its isolation in Japan, it was never marketed. Thus, the first statin drug available to the public was made from lovastatin, a secondary metabolite produced by Aspergillus terreus. This type of fungi common grows on carbon-rich substrates such as monosaccharides, so they are often contaminants of starchy foods such as bread. A. terreus is also used to produce important organic acids, such as itaconic acid and cis-aconitic acid.
Lovastatin was isolated in 1978 by Alberts, Chen and others at Merck Research Laboratories. After many years of clinical trials and some controversy over animal toxicity, lovastatin became available for prescription in 1987. By 2002, the Heart Protection Study confirmed the safety of simvastatin (which is obtained from lovastatin by ester hydrolysis and re-esterification) and also demonstrated the clinical benefit in 20,000 patients. In addition to simvastatin, other statin drugs include pravastatin, which is isolated using Streptomyces carbophilus by microbiological hydroxylation, atorvastatin, fluvastatin, and rosuvastatin. These statin drugs are now considered to be dramatically effective for lowering LDL cholesterol with no obvious adverse effect.
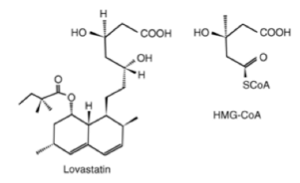
Hypercholesterolemia, or elevated levels of LDL cholesterol, is related to an increased incidence of cardiovascular disease, and lowering LDL cholesterol levels can significantly reduce the incidence of this health risk. Cholesterol is biologically synthesized in the liver and kidney from acetyl-CoA by the hydroxymethylglutaryl-CoA (HMG-CoA) reductase or the mevalonate pathway. Through a series of reactions, acetyl CoA is reduced to mevalonate by the enzyme HMG-CoA reductase and this reduction is the rate-limiting and irreversible step in cholesterogenesis. A part of the compound mevastatin and the other structurally similar family of statin drugs resembles HMG-CoA, which suggests that these compounds decrease the production of cholesterol through competitive inhibition. Unlike HMG-CoA, both mevastatin and lovastatin can form lactones by elimination of water from the carboxylic acid group on C-1 and the OH group on C-5 of both structures. The water soluble, open-chain forms of these drugs are more pharmalogically potent. In the open ring forms, these statins which mimic the half-reduced substrate mevaldate hemithiacetal during the reduction of HMG-CoA to mevalonate have a high affinity towards HMG-CoA reductase (104 greater than that of normal substrate).
However, the mechanism of the reduction in cholesterol by statins is not simply the decrease in cholesterogenesis. In the 1970s, Brown and Goldstein realized the central role of the hepatic LDL receptor in determining the concentration of LDL cholesterol in plasma. Inhibition of HMG-CoA reductase reduces the levels of mevalonate, and thus results in a decrease in the amount of the regulatory steroids. This upregulates the production of HMG-CoA reductase and of the LDL receptor. Although the LDL receptor was not the original target that Alberts and Chen believed lovastatin was acting on, Brown and Goldstein proved that the activation of the receptor is important for the statins to control cholesterol production.
Currently, statins are routinely prescribed to prevent coronary artery disease. In addition, they are being researched for anti-inflammatory and anti-cancer properties as well as for potential drugs against Alzheimer’s disease and osteoporosis.
(1)Daily Finance. The 10 Biggest-Selling Drugs That Are About to Lose Their Patent http://www.dailyfinance.com/2011/02/27/top-selling-drugs-are-about-to-lose-patent-protection-ready/ (accessed 2/14/12).
(2)Barton, D.; Nakanishi, K. Comprehensive Natural Products Chemistry, Vol 2. Isoprenoids including carotenoids and steroids. Elsevier Science Ltd: UK, 1999; Vol.2, pp 25.
(3)Tobert, J. Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors. Nature Reviews Drug Discovery. 2003, 2, 517-526.
(4)Dewick, P.M. Medicinal Natural Products, 3rd ed.; John Wiley & Sons Ltd: UK, 2009; pp 98.
"Statins are to cardiovascular disease what penicillin was to infectious disease. They are one of the most important, if not the most important, advances in cardiovascular medicine."
- Dr. Leon Simons, Stanford
Thursday, February 16, 2012


Statins - Maya Heck